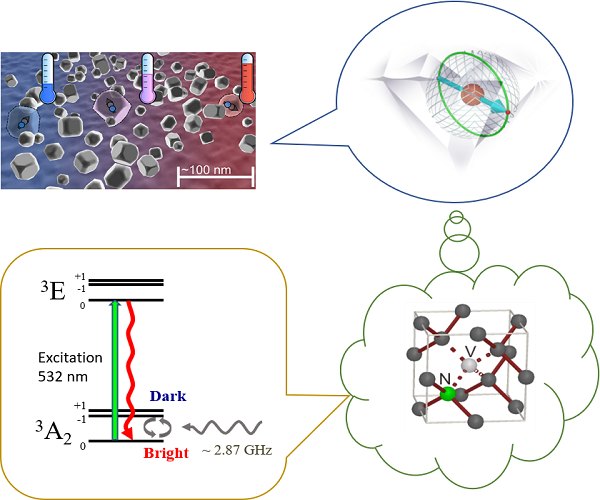
Diamonds are not only shining but also colorful. There are more than 500 kinds of diamonds in nature, all caused by structural defects or impurities in the diamond crystal. For instances, blue diamond contains boron, yellow diamond contains nitrogen, and green diamond is due to voids in the lattice; perfect diamond crystal is transparent and colorless in the range of visible light.
Diamonds are classified into two major categories, and the most abundant one is type I diamond which contains nitrogen as the major impurity. In particular, type Ib diamond contains single nitrogen atoms dispersed in isolated lattice sites, which gives the diamond a yellowish color. A single nitrogen atom, when combined with a lattice vacancy, forms a light emitter which fluoresces in red when excited by green or blue light. This defect, known as the nitrogen-vacancy color center (NV center), is often viewed as an artificial atom trapped in the diamond crystal, not only because of its atom-like simple electronic structure but also its extraordinary long quantum coherence time even in room temperatures. The ability to detect and manipulate its quantum coherence enables novel application in quantum information and sensing.
In this poster we will introduce how nano-diamonds containing high concentration of NV centers can be implemented into nano-sensors for measuring temperatures, static magnetic fields, and time-varying (microwave) magnetic fields. Nano-sensors can be utilized to measure the environmental parameters of a tiny object under investigation and study microscopic physics therein.
